The post ANSI/AAMI ST108 Summary: Water for the Processing of Medical Devices appeared first on ChemTreat, Inc..
]]>ANSI/AAMI ST108 Water for the processing of medical devices was approved on August 16, 2023. This standard replaced the Technical Information Report 34. Unlike the Technical Information Report, the updated standard provides clear requirements for every stage of medical device processing. ANSI/AAMI ST108 establishes minimum requirements for water quality and steam purity for processing medical devices intended for patient use. Proper implementation of this standard improves the effectiveness and lifespan of equipment, such as ultrasonic sterilizers, endoscope reprocessors, autoclaves, and local steam generators. Implementing this standard involves a comprehensive process, starting with an assessment of current water quality management practices.
Standard Applicability
The applicability of ANSI/AAMI ST108 applies to facilities that have medical device processing equipment (cleaning, rinsing, disinfection, and sterilization). Criteria includes the considerations listed below.
Water is categorized into three different types based on the disinfection characteristics or sterilization process type.
a. Utility water is tap water that is used for rinsing, flushing, and intermediate rinsing (rinsing between cleaning and disinfection). This water may require treatment.
b. Critical water is final rinse water that has been processed by highlevel disinfection. To meet the water quality requirements, critical water requires an extensive multi-step treatment process. This process can include pretreatment, storage, distribution, and final treatment.
c. Steam is considered vaporized water produced by a centralized boiler near the sterilizer(s). Steam is tested as condensate, following the sterilizer.
A multidisciplinary team must be established and should have knowledge of the water systems and associated processes in use. Team members should include facilities engineering staff, infection prevention, medical device processing personnel, clinical engineering staff, and water treatment specialists.
A risk analysis should be performed to evaluate and identify risks associated with utility water, critical water, and steam. The following water characteristics should be considered: physical appearance, microbial concentrations, inorganic/organic contaminants, pH, conductivity, and temperature.
Routine monitoring and performance qualification testing is essential to maintaining the integrity of the water treatment systems. The updated standard provides detailed guidance on water quality testing standards, testing frequency, and system functionality. Monitoring is performed by the multidisciplinary team. The team reviews the water quality results and implements the necessary corrective actions if the results are out of range. The standard includes reference tables that specify testing parameters and frequencies.
The new standard provides guidance on water treatment system installation, operation qualification, and design of water treatment systems. It also provides recommendations on service interruptions, system shutdowns, and construction related activities.
The standard serves as a framework for facilities to create an impact on water quality and patient safety. Proper implementation of this standard can help prevent issues such as, medical device and processing equipment damage, processing inefficiencies, and negative patient outcomes from surgical site infections.
To access the standard, go to: https://www.aami.org
Download the PDFThe post ANSI/AAMI ST108 Summary: Water for the Processing of Medical Devices appeared first on ChemTreat, Inc..
]]>The post Air Separation Plant Reduces Azole Discharge, Heat Exchanger Corrosion, and Chemical Costs with MultiCuRE™ appeared first on ChemTreat, Inc..
]]>A large industrial air separation company in the Southern US was feeding an azole-based product to inhibit corrosion in their brass and mild steel heat exchangers.
The plant was looking for an alternative treatment program to reduce azole discharge into local waterways while improving corrosion rates.
The Solution
ChemTreat began implementing proprietary MultiCuRE, a low-azole corrosion inhibitor, which works by forming a hydrophobic, halogen-resistant film on metal surfaces to reduce corrosion.
The product was base fed via mass balance and verified with an on-line azole residual detection probe. Treatment performance was monitored with corrosion coupons and on-line corrators.
The Results
Containing 80% less azole than the incumbent treatment program, MultiCuRE enabled the air separation plant to reduce azole discharge, helping them meet their environmental goals.
The improved cost performance of MultiCuRE over the azole-based program allowed the facility to reduce water treatment chemical spend.
The plant also saw a significant improvement in corrosion rates. Brass corrosion decreased from 2.1 to 0.4 mpy, and mild steel corrosion decreased from 2.3 to 1.6 mpy.
5X brass corrosion reduction
1.4X mild steel corrosion reduction

Corrosion coupon showing the hydrophobic film formed by MultiCuRE
Based on the product’s positive impact on environmental goals and chemical costs, the company continued using MultiCuRE after the initial trial ended and plans to implement it at other facilities.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Air Separation Plant Reduces Azole Discharge, Heat Exchanger Corrosion, and Chemical Costs with MultiCuRE™ appeared first on ChemTreat, Inc..
]]>The post Highwire Contractor Success Platform Recognizes ChemTreat with Platinum Safety Award appeared first on ChemTreat, Inc..
]]>“Management systems are critical to any company’s ability to deliver successful outcomes and, most importantly, to keep their employees safe,” said David Tibbetts, CSP, Highwire’s Chief Safety Officer. “ChemTreat, Inc. has done a remarkable job implementing a strong safety management system resulting in exceptional safety performance and results.”
Highwire’s Safety Assessment reviews a company’s historic and current safety performance. The program provides a thorough, objective, and consistent evaluation of company performance so clients and contractors can identify, monitor, and mitigate risks more effectively. The results provide a strong indicator of how a contractor values safety and serve as a reliable predictor of future performance.
“Highwire’s Platinum Safety Award is a validation of our commitment to drive towards zero safety incidents. Whether it is within our own production facilities or at the customer’s site, protecting our associates is one of our highest priorities,” said ChemTreat VP of Marketing Lori Palmer. “Safety is at the heart of our business system processes that underpin our culture of continuous improvement.”
The Platinum Safety Award is presented to companies who score between 95 and 100 on Highwire’s Safety Assessment and is only achieved by the top 5% of Highwire contractors.
To view ChemTreat’s Highwire Network Profile, click here.
The post Highwire Contractor Success Platform Recognizes ChemTreat with Platinum Safety Award appeared first on ChemTreat, Inc..
]]>The post Direct-to-Chip Liquid Cooling Treatment Helps DOE National Laboratory Improve System Performance appeared first on ChemTreat, Inc..
]]>National laboratories managed by the Department of Energy (DOE) often have extensive direct-to-chip liquid cooling and high-performance computing (HPC) systems that need to operate at peak efficiency to meet critical research goals. These facilities require a variety of customized water treatment solutions to maintain operating efficiency and preserve their assets.
One national laboratory in the Southern United States was struggling with performance issues in its open recirculating, closed loop, and HPC cooling systems. As a preferred water treatment consultant to the DOE, ChemTreat employed state-of-the-art technologies and provided technical and analytical support to address the lab’s needs.
Improving Cooling System Performance
Addressing Inefficiencies in Open Recirculating Cooling Towers
Project Description
The laboratory operates several large open recirculating water systems responsible for cooling critical research equipment and data center HPCs. These systems were fouled with mineral deposits that significantly impacted heat transfer and cooling efficiencies and contributed to increased metal corrosion and microbiological activity.
Solution
To address these issues, ChemTreat’s Research & Development team developed a treatment solution using Quadrasperse® polymeric dispersant and corrosion inhibition technologies, specifically designed to meet the unique needs of this facility.
Results
After applying our custom-designed treatment program, mineral deposits were removed from the cooling tower fill and heat exchanger surfaces, significantly improving heat transfer efficiency and reducing the risk of underdeposit corrosion and microbiological activity.
Enhancing HPC Cooling Performance
Project Description
The facility has many computer applications with direct-to-chip liquid cooling, including a large, recently built HPC that was unable to pass speed testing because of poor cooling performance.
The HPC’s cooling loops had become heavily fouled with biological growth, mineral scale, and other additives, preventing the unit from reaching the maximum expected calculations per second that it was designed to achieve.
Traditional cooling treatment methods add film inhibitors, such as silicates, to control corrosion, and biocides to mitigate microbiological issues. However, direct-to-chip liquid cooling systems have slightly different needs.
Solution
ChemTreat developed CL2001, a product specifically designed for CPU applications, to treat the laboratory’s HPC cooling loops. This treatment methodology takes a more holistic approach, avoiding inhibitors that can cause fouling while implementing a proprietary protocol to control corrosion and microbiological activity.
Results
The CPU cooling at the site has been optimized with CL2001 treatment, and the HPC has passed the speed test. Stress testing is ongoing, and the laboratory is expected to accept ownership of the HPC from the manufacturer in the coming months.
Addressing Fouling and Corrosion in Aluminum Closed Loop Cooling Systems
Project Description
The closed loop system was experiencing fouling events that led to underdeposit corrosion in aluminum equipment, reducing heat transfer efficiency.
Solution
The facility began using ChemTreat’s patented FlexPro® multi-metal corrosion inhibitor specifically designed for aluminum applications. The new inhibitor has a neutral pH, allowing the cooling systems to operate within an acceptable pH range.
Results
Thanks to the application of the patented FlexPro corrosion inhibitor, the chilled water loop is now free from suspended solids caused by corrosion byproducts. The water clarity is excellent, and heat transfer and cooling efficiency have improved greatly. Aluminum corrosion has been reduced from >10 mpy to <0.1 mpy.
Value-Added Water Conservation Project
In addition to treating the issues experienced by the laboratory’s closed loop cooling systems, ChemTreat implemented a program to help the facility meet environmental goals.
Utilizing a proprietary, state-of-the-art aluminum corrosion inhibitor, ChemTreat assisted the laboratory with a once-through to closed loop cooling water conservation project, saving 34 million gallons of water per year while protecting critical research equipment.
Conclusion
ChemTreat’s water treatment expertise and custom solutions helped a national laboratory improve the efficiency of its direct-to-chip liquid cooling and other cooling systems.
The results of this partnership have yielded the following benefits for the facility:
- Cooling tower systems have remained free of mineral deposits and have exhibited excellent corrosion and microbiological control for more than a decade.
- Improvements to CPU cooling treatment helped the facility’s HPC pass the speed test.
- Aluminum corrosion reduction in the closed loop improved water quality and heat transfer efficiency.
- A water conservation project in the closed loop cooling system helped the laboratory save 34 million gallons of water annually.
Results are examples only. They are not guaranteed. Actual results may vary.
The post Direct-to-Chip Liquid Cooling Treatment Helps DOE National Laboratory Improve System Performance appeared first on ChemTreat, Inc..
]]>The post Combined Cycle Power Plant Reduces Condensate Cation Conductivity with Non-Amine Filming Technology appeared first on ChemTreat, Inc..
]]>A 2×1 combined cycle power plant in the Northeast US ceased baseload operation and began cycling because of fluctuating power prices. This resulted in varying periods of layups that could last up to two weeks, increasing corrosion potential in the steam and feedwater systems of their two heat recovery steam generators (HRSGs). The facility also saw an increase in iron transport during each startup.
The plant began feeding a traditional filming amine to manage steam system corrosion. While it helped in reducing iron transport, the product increased cation conductivity levels in the condensate system, potentially masking condenser tube failures and demineralizer leakage.
Non-amine filming technologies are known to maintain low cation conductivities while delivering the same corrosion performance as traditional filming amines. Efforts to reduce their cation conductivity levels led the plant to explore non-amine filming options.
The Solution
ChemTreat recommended non-amine filming product BL9000, designed to reduce system cation conductivity levels while helping plants manage corrosion in their HRSGs during layup conditions by limiting the amount of iron transport through the system during startups.
BL9000 was applied to the feedwater of both HRSG units over a period of three months, during which the plant saw several periods of cycling and layup. The product was fed at a consistent dosage, with condensate cation conductivity levels monitored during plant operation. Additionally, iron levels were monitored during startups using Millipore filter tests.
At the end of the three-month period, the plant went into an outage, and the HRSG steam drums were inspected to evaluate the efficacy of the BL9000 treatment program.
The Results
During the trial period, a significant decrease in cation conductivity (0.7–0.8 µmho) was observed, allowing the plant to better monitor for condenser leaks and evaluate the effectiveness of the new program.

0.7 µmho decrease in cation conductivity observed during the facility’s longest run period at the time of the BL9000 trial.
Throughout the three-month trial period, Millipore filter tests conducted during plant startups consistently showed less than 10 ppb iron in the high-, intermediate-, and low-pressure drums as well as the condensate system.

Millipore filter test results showing <10 ppb iron for two consecutive plant startups
As one of the key performance indicators (KPIs) for film-forming product effectiveness, this result demonstrates continued effectiveness in steam system corrosion protection during layups, which can decrease downtime associated with equipment failures.
The steam drum inspection showed that BL9000 had formed a protective film, causing water to bead on the mild steel surfaces within the steam drums, thus reducing iron corrosion.

BL9000 protective film formation on the mild steel surface within the low-pressure steam drum.
Based on the significant reduction in cation conductivity and the continued low levels of iron detected during startups, the plant has elected to adopt BL9000 non-amine technology as their preferred treatment program for their HRSGs.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Combined Cycle Power Plant Reduces Condensate Cation Conductivity with Non-Amine Filming Technology appeared first on ChemTreat, Inc..
]]>The post ChemTreat Named Industrial Supplies & Services Supplier of the Year by Molson Coors Beverage Company appeared first on ChemTreat, Inc..
]]>For over 30 years, ChemTreat and Molson Coors have been working together to develop and launch sustainability initiatives, cost reduction efforts, and other water treatment projects at multiple Molson Coors facilities across the US.

(From left to right) ChemTreat’s Mark Johnson (Senior Sales Leader), Joe Keating (VP, Global Corporate Accounts & South America Sales), Jacob Paugh (Strategic Account Manager), and Phil Pilon (Senior Director, Global Light Industry) at the Molson Coors Supplier of the Year Awards Ceremony
In 2023, ChemTreat was chosen to play an integral role in a number of sustainability initiatives at Molson Coors. This includes two wastewater reclamation projects that, when completed, will allow support increased recovery and reclamation of hundreds of millions of gallons of water annually, helping support Molson Coors’ company’s water use efficiency goals.

ChemTreat has also implemented sustainability audits at other Molson Coors breweries, working with local teams to identify further opportunities for water savings.
“ChemTreat and Molson Coors have proven what the potential of a true partnership based on trust and performance can achieve,” said Jacob Paugh, Strategic Account Manager at ChemTreat.
This award was presented to the ChemTreat team on May 7 at the Molson Coors global headquarters in Milwaukee, Wisconsin.

The ChemTreat team was honored by Molson Coors at the Supplier of the Year Awards Ceremony on May 7, 2024
We look forward to continuing our partnership with Molson Coors to identify and implement further water treatment initiatives that contribute to the efficiency and sustainability goals of the brewing company and advance our efforts to Maximize the Power of Water®.
The post ChemTreat Named Industrial Supplies & Services Supplier of the Year by Molson Coors Beverage Company appeared first on ChemTreat, Inc..
]]>The post ChemTreat Recognizes Air Liquide with Power of Water Award appeared first on ChemTreat, Inc..
]]>At the Bayport facility, Air Liquide and ChemTreat collaborated on an initiative called “Runtime Extension and Lower Environmental Impact Project Using Chlorine Dioxide for Disinfection Treatment of the Reverse Osmosis Plant.” The teams worked together on enhancing the treatment of plant supply water to optimize water consumption and maintain system cleanliness while extending runtime between cleanings.
By implementing a chlorine dioxide-based treatment process, Air Liquide was able to save over 273,000 gallons of water annually while significantly reducing chemical usage. This program improved the efficiency of the plant while helping Air Liquide meet their environmental goals around water savings.
“Air Liquide is honored to be presented the 2024 Power of Water Award by ChemTreat,” said Andrew Garnett, President, Air Liquide Large Industries North America. “The collaboration between Air Liquide and ChemTreat to continuously innovate sustainability initiatives that benefit the environment is a direct reflection of our fundamental responsibility to environmental performance. Thank you to the Bayport facility and our procurement teams for your environmental leadership.”

Members of ChemTreat’s team presented the Power of Water Award to Air Liquide in an Earth Day ceremony.
At the Power of Water Award Ceremony, which took place on Earth Day (April 22, 2024), Paul Guidotti, ChemTreat’s VP of Sales & Service commented, “Air Liquide demonstrated many dimensions of excellent performance through their chlorine dioxide treatment program to save over 273,000 gallons of water annually. ChemTreat is very pleased with what we’ve accomplished together in the past year with Air Liquide and look forward to our continued collaboration in the future.”
Air Liquide’s ADVANCE strategic plan lays out the company’s commitment to the environment and the important role of water management in inventing a more sustainable future. The Group aims to implement a water management plan and define a global standard for the quality of discharged water by 2025.
ChemTreat is proud to work together with customers like Air Liquide on projects that yield tangible, measurable benefits for the environment as part of our mutual commitment to sustainability and Maximizing the Power of Water®.
About the Power of Water Award
Launched in 2022, the ChemTreat Power of Water Award was designed to recognize a project or innovation that demonstrates the greatest positive impact on environmental sustainability. Through both their own expertise and partnership with ChemTreat, recipients of this prestigious award have achieved excellence in environmental stewardship and sustainability, demonstrating environmental leadership in their industry.
The post ChemTreat Recognizes Air Liquide with Power of Water Award appeared first on ChemTreat, Inc..
]]>The post Hospital Improves Cooling Treatment Program Storage and Handling While Meeting Environmental Goals appeared first on ChemTreat, Inc..
]]>A hospital in Virginia was using a traditional liquid cooling treatment program to manage corrosion, deposition, and fouling in their system. However, the presence of large containers of corrosive chemical on-site was causing storage and handling concerns.
The Solution
Based on recommendations from ChemTreat, hospital personnel decided to switch to Smart Release® solid cooling treatment technology to reduce chemical handling and storage requirements while maintaining system corrosion, scale, and fouling at appropriate levels to preserve assets and system efficiency.
The Results
The Smart Release inhibitor treatment system was installed with canister feeders to apply the solid products, replacing the liquid cooling corrosion inhibitors and oxidizing and nonoxidizing biocides.

Switching to Smart Release® provided the following benefits to the hospital:
- The patented polymer coating on Smart Release tables controls the release of chemical, so product is fed only when the cooling tower is running and the feeder has flow. When flow stops, the osmotic pressure gradually equalizes to pause chemical feed. This enables treatment to be applied without using electrical pumps, saving on energy use.
- Transportation fuel consumption was reduced, as over 650 pounds of liquid cooling treatment were replaced with 100 pounds of Smart Release chemical.
- With 100% recyclable packaging and feeders made of recycled materials, the Smart Release program helped the hospital achieve environmental goals. Smart Release even improved the facility’s LEED rating.
- The consistency of product feed reduced the frequency of testing, freeing up personnel for other projects.
Based on the success of this program, hospital personnel decided to convert five other sites to Smart Release.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Hospital Improves Cooling Treatment Program Storage and Handling While Meeting Environmental Goals appeared first on ChemTreat, Inc..
]]>The post ChemTreat Helps Medical Facility Solve Instrument Corrosion Issues appeared first on ChemTreat, Inc..
]]>A hospital system had a very serious problem with staining and corrosion on reusable medical instruments caused by the cleaning and sterilization process. This particularly affected specialized knives. The problem was so severe that surgeries were interrupted, postponed, and even canceled until properly prepared instruments could be made available to the surgeons.
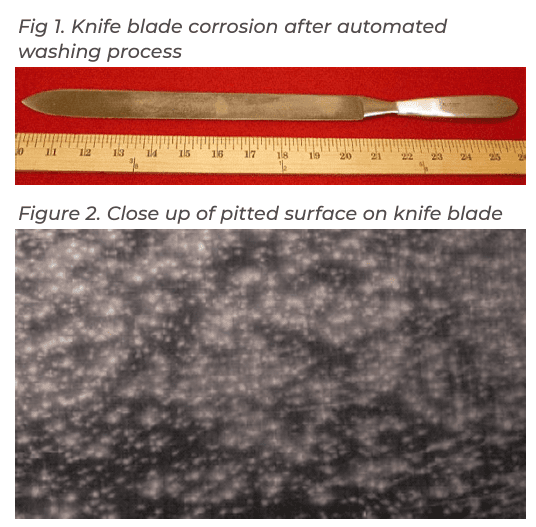
Figure 1 shows a corroded amputation knife after going through the automated washing process prior to sterilization. Since the knife blade was corroding but not the handle, it was determined the chromium content of the blade was not sufficiently resistant to corrosion. In addition to pitting corrosion, the surface was found to have clusters of mineral deposits.
The first three of the four automated washing stages used unsoftened tap water. The final rinse stage used demineralized water, but only rinsed for ten seconds.
The Solution
Based on ChemTreat’s recommendations, the facility instituted the following changes in the wash cycle:
- More attention paid to the pre-wash steps.
- Purified water incorporated earlier in the washing procedure.
- Water source quality maintained at appropriate levels to reduce corrosion.
ChemTreat also recommended switching to blades made with a more corrosion-resistant alloy.
Thanks to these improvements, the facility was able to solve their issues with medical instrument corrosion while improving the sterilization process overall.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post ChemTreat Helps Medical Facility Solve Instrument Corrosion Issues appeared first on ChemTreat, Inc..
]]>The post University Medical Complex Saves $500k in Annual Water Costs with ChemTreat Program appeared first on ChemTreat, Inc..
]]>A major university medical complex needed to replace more than a dozen aging cooling towers and add ten new buildings, all while transitioning to a centrally located chiller plant.
As part of this new system, the facility wanted makeup water to come from five sources, each with varying water quality properties:
- City water
- Available creek water
- Collected rainwater
- Retrieved condensate
- Reused, RO-filtered blowdown water
Facility personnel were also looking to save on costs and improve the sustainability of their water treatment program by extending the life of RO membranes and pre-filter cartridges, improving system monitoring, and reducing scale and corrosion.
The Solution
The previous process was not capable of handling a new 32,000-ton centralized plant using multiple makeup water sources.
The university partnered with ChemTreat for in-depth expertise and on-site support with this project.
After a thorough system evaluation, the ChemTreat team provided a list of recommendations and worked with the university to implement the improvements.
ChemTreat’s recommendations included:
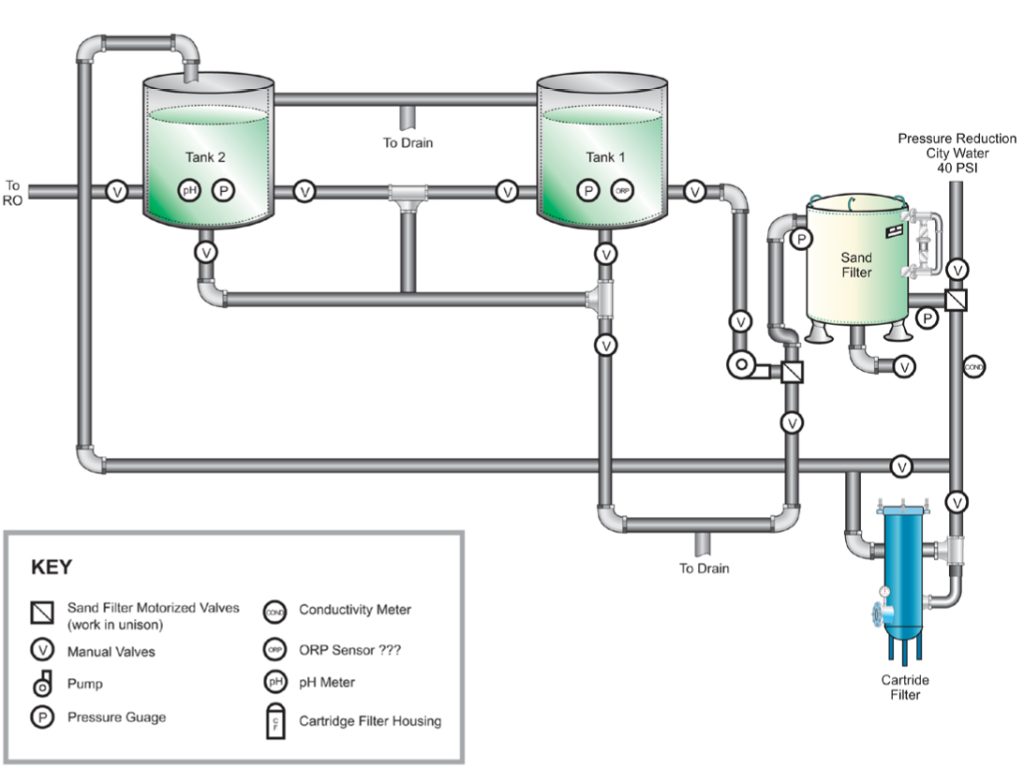
- Repositioning a sand filter in relation to the RO filtration system.
- Adding 1-micron filters to supplement the 5-micron filters for suspended solids removal (a limiting factor for RO membrane efficiency) to extend filtration run duration between cleanings.
- Implementing CTVista® intelligent water management software to solve communication issues.
- Enhancing the cooling treatment program to manage the variable water quality of five different makeup sources.
The Results
Redesigned Pretreatment Process
- Pretreatment cartridge life extended from three days to over a month.
- Off-line cleaning times reduced from five hours four times per week to just three hours every three weeks, reducing downtime, system disruptions, and costs.
CTVista Monitoring System Implementation
Thanks to the installation of CTVista, all team members with data access could write reports and other observations within a centralized system, allowing all program participants to review data and determine the appropriate course of action.
Cooling Treatment Program Enhancements
- Biocide program improvements reduced bacteria counts from 103 cfu/mL to 102 cfu/mL.
- A consistent corrosion rate of <0.1 mpy established.
- Conductivity maintained at a consistent level throughout multiple water sources.

Water Savings
With ChemTreat’s support, the university was able to save over $500,000 per year in water costs thanks to improvements to its water treatment processes.
Annual water savings from reclamation projects:
- >12 million gallons of condensate
- >20 million gallons of creek water
- >25 million gallons of recovered blowdown
Results are examples only. They are not guaranteed. Actual results may vary.
The post University Medical Complex Saves $500k in Annual Water Costs with ChemTreat Program appeared first on ChemTreat, Inc..
]]>The post ChemTreat Recognized with Platinum Safety Award from Highwire appeared first on ChemTreat, Inc..
]]>“Highwire’s Platinum Safety Award is a validation of our commitment to drive towards zero safety incidents,” said Lori Palmer, VP of Marketing at ChemTreat. “Whether it is within our own production facilities or at the customer’s site, protecting our associates is one of our highest priorities. Safety is at the heart of our business system processes that underpin our culture of continuous improvement.”
The Platinum Safety Award is presented to companies who score between 95 and 100 on Highwire’s Safety Assessment and is only achieved by the top 5% of Highwire contractors.
“Management systems are critical to any company’s ability to deliver successful outcomes and, most importantly, to keep their employees safe. ChemTreat, Inc. has done a remarkable job implementing a strong safety management system resulting in exceptional safety performance and results,” says David Tibbetts, CSP, Highwire’s Chief Safety Officer.
Highwire’s Safety Assessment reviews a company’s historic and current safety performance. The program provides a thorough, objective, and consistent evaluation of company performance so clients and contractors can identify, monitor, and mitigate risks more effectively. The results provide a strong indicator of how a contractor values safety and serve as a reliable predictor of future performance.
View ChemTreat’s Highwire Network Profile here.
About ChemTreat: ChemTreat’s expertise in water and process treatment solutions spans a variety of industries, including data centers, hospitals, and universities. With an experienced team of technical and field representatives, ChemTreat has developed a robust portfolio of products and services that helps facilities maintain and enhance operational efficiency while meeting their environmental goals.
The post ChemTreat Recognized with Platinum Safety Award from Highwire appeared first on ChemTreat, Inc..
]]>The post RO Makeup Water Sources: Addressing Variable Water Quality to Maintain System Efficiency appeared first on ChemTreat, Inc..
]]>This article discusses the characteristics of various makeup sources and outlines processes for analyzing RO makeup water.
Read on to:
- Examine the various types of makeup water sources.
- Explore the impact of minerals, organics, microbiological growth, and other constituents on RO systems.
- Learn some best practices for maintaining RO system efficiency.
Makeup Water Sources: Where is the Water Coming From?
To understand the potential vulnerabilities of an RO system, it is important to know its makeup water source.
RO manufacturers typically suggest cleaning systems every 3–4 months; yet, in many cases, RO systems require more frequent cleanings. This is, in part, because of the differences in water coming from varying makeup sources, and more importantly, the performance of the upstream filtration equipment. Pretreatment is a key component of reliable and efficient RO.
Makeup water quality is very important. Any imperfections in the water can lead to fouling, scaling, and corrosion if not treated properly.
Surface Water
Surface water is drawn from sources such as:
- Rivers
- Lakes
- Reservoirs
- Canals
Typically, all surface waters contain fluctuating levels of suspended and colloidal particles, organics, microbiological activity, and turbidity. If the makeup source is a river, for example, the clarity or turbidity will change based on rainfall and land runoff.
Surface water contains suspended and colloidal particles, which will pass through a 5-micron cartridge filter in the RO, increasing pressure changes (ΔP). This can result in RO fouling during the first stage. Conducting a Particle Size Analysis of the makeup water and the outlet of the cartridge filter housing is recommended at this stage.
Well Water
Another source for makeup is well water.
- Shallow wells are typically located anywhere from 40 to 150 feet below the surface.
- Deep wells can be found anywhere from 600 to 1,000 feet below the surface.
Generally, the deeper the well, the cleaner the water. Deep wells have more consistent temperatures, and water pulled from these wells often contains lower levels of bacteria, colloidals, organics, and suspended particles.
Gray Water
Gray water typically refers to tertiary wastewater intended for municipal and industrial reuse.
In recent years, water scarcity has made gray water a more popular source for makeup. Instead of using surface water, many municipalities now use gray water from power plants, chemical plants, and refineries for their processes.
Although gray water use has gained in popularity, it comes with unique treatment challenges. Gray water typically has a large amount of organic loading, ammonia, and phosphates, while its low chlorine residual is rarely sufficient for removing microbio.
How to Maintain RO Efficiency with Makeup Water Analysis
Silt density index (SDI) testing is a best practice for analyzing RO makeup, as it measures the fouling capacity of the water used in the RO.
SDI testing is performed on site with an SDI kit. The test requires a constant makeup stream at 30 psi. This stream is passed through a 0.45-micron filter pad at a known volume for 15 minutes to calculate SDI.
Typically, RO manufacturers recommend an SDI of 5 or below, but many are now indicating that 3 or less is needed for good RO performance.
In most cases, the lower the SDI, the better the system will run. It is a good idea to save the SDI pads for historical reference.
Upstream Chemical Addition to Surface Water Makeup
Utilizing surface water as a makeup source requires facilities to implement either a clarification process to remove turbidity or a cold lime softening process to reduce hardness, alkalinity, and turbidity.
Regardless of which process is implemented, treatment will include coagulant and/or flocculant chemicals.
Coagulants
Coagulants have one of three bases:
- Aluminum
- Iron
- Organic
The amount of coagulant fed to the system greatly varies based on the surface water source.
A good example of this is the Mississippi River, which flows north to south. At the top of the river in Minnesota, the turbidity level of the water is low, so coagulant feed will be in a low parts per million (ppm) range.
As it moves south, the Mississippi meets the Missouri, Illinois, and Ohio rivers. These confluences lead to turbidity formation, so water treatment plants along the river feed higher amounts of coagulant into their system to adjust for higher turbidity.
Flocculants
Flocculants also play an important role in turbidity reduction. They can be cationic, anionic, or non-ionically charged. Flocculants are typically dosed at less than 2 ppm, so it is important to monitor how flocculants are being added to your system.
The Challenges of Coagulant and Flocculant Treatment
Though coagulants and flocculants are beneficial for producing the desired finished water quality, they can still be detrimental to overall RO membrane health.
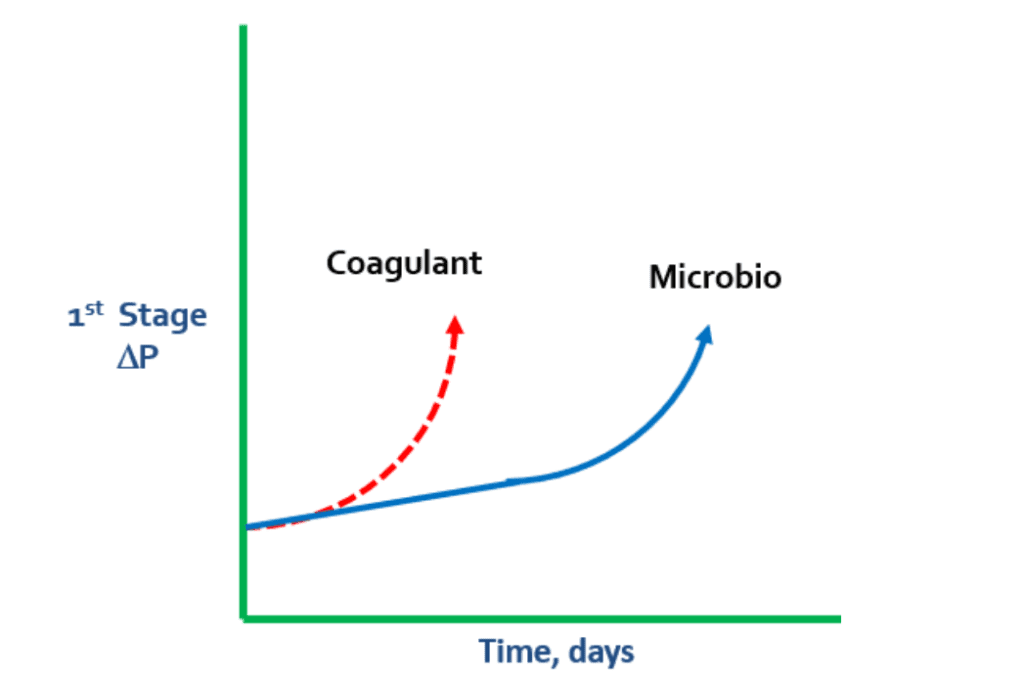
Unlike the slightly negatively charged RO membranes, coagulants are cationically charged, which can cause pressure changes. If there is a rapid spike in ΔP during the first stage, it is prudent to look for a coagulant problem. Coagulant levels may not have been adjusted to match changes in turbidity, which can result in coagulant overfeed.
In these cases, coagulant begins to lay down on the membrane and grab microbio, particles, and colloidals. When the negatively charged bacteria meets the positively charged coagulant, the coagulant continues to grab more and more microbio.
In some cases, extreme levels of polysaccharides in microbio can cause a “flypaper” effect, which leads to microbiological matter grabbing suspended solids and colloidals from the water. This can result in membrane fouling.
If the RO membrane is not cleaned properly, coagulant issues can lead to gap formation.
Once gaps form inside an RO membrane, they are very difficult to remove. Therefore, it is important to monitor microbio levels within the makeup water as it enters the system.
Additionally, if a plant is using city water for makeup, it is recommended that facility personnel contact the municipality and ask what type of coagulant has been used upstream.
The Impact of Minerals on Makeup Water
It is also important to be aware of the mineral contents of makeup water.
Minerals can be broken down into two separate subgroups: cations and anions.
Examples of each are included below:
| Cations | Anions |
|---|---|
| Calcium | Total Alkalinity |
| Magnesium | Chlorides |
| Sodium | Sulfates |
| Potassium | Nitrates |
| Barium | Fluoride |
| Strontium | Orthophosphate |
| Aluminum | Silica |
| Iron | |
| Manganese |
Testing makeup water for mineral content is an essential step in determining the type of RO antiscalant to use and the percent RO recovery rate.
Of the cations, barium, aluminum, iron, and manganese are particularly important to monitor. Levels of each should typically not exceed 0.05 ppm (50 ppb).
Aluminum can be especially problematic. It is typically fed as a coagulant to reduce turbidity, but excess aluminum can lead to RO fouling, which can be difficult to treat with an antiscalant.
The following anions: total alkalinity, orthophosphate, fluoride, and silica, may also need to be monitored closely. Gray water is particularly prone to supplying excess phosphates.
The recommended testing frequency for minerals in makeup is:
- Every other month for surface water sources
- Quarterly for well water
- Monthly for gray water
In general, calcium phosphate, calcium sulfate, and barium sulfate can all be treated with an antiscalant, while aluminum, iron, and manganese may be a bit more difficult treat.
Other Important Testing Parameters
pH
Once the pH and total alkalinity are known, the amount of free carbon dioxide (CO2) can be calculated.
Because it is a gas, CO2 passes through the RO membrane, which can lead to pH reduction in the permeate.
Since CO2 is not detected by a conductivity meter, it can flow downstream to the mixed bed and result in a large amount of hidden loading. This could require more frequent replacement of the mixed bed.
Conductivity
Calculating conductivity provides an idea of the number of ions present in the water. For example, high conductivity (300–500 µS) may indicate that hardness and alkalinity levels are within range, but there may still be a high number of chlorides or sulfates in the water. High conductivity may also suggest that more permeate is traveling downstream to the softener or other ion exchange unit.
Total Organic Carbon (Organics)
Surface waters are notorious for having high levels of organic matter, which is measured as total organic carbon (TOC). For RO feedwater, the TOC limit is 3 ppm.
- TOC can be natural or synthetic:
- Natural organics
- Tannin, Lignin, Humic, etc.
- Typically yellowish to brownish in color
- Great source of nutrients for bacteria
- Synthetic organics
- Often coming from farm runoff chemicals
- Can have a greater fouling effect on RO membranes compared to natural organics
- Natural organics
Organics with a molecular weight of 150–200 ppm and higher are rejected by the RO; however, a certain percentage of organics below that molecular weight will pass through the RO into the permeate.
Temperature
Temperature affects the flux rate for permeation. Cooler temperatures cause membrane pores to tighten, decreasing the flux rate. Warmer water loosens up the pores, allowing more total dissolved solids (TDS) or ions to pass through.
Orthophosphates in gray water or calcium in industrial reuse makeup streams can form calcium phosphate (a sludge-like deposit) in the last stage of the RO as the water gets warmer.
The solubility of silica and the flux, on the other hand, decrease as water temperature goes down, and vice versa.
Chlorine
Chlorine degrades RO membranes and needs to be removed either with uncatalyzed sodium bisulfite or an active carbon filtration system. If a transitional metal, such as iron, manganese, or cobalt, is present in a system containing chlorine, the rate of membrane degradation is accelerated.
Often, active carbon beds are paired with a biocide program. The two most commonly used in the industry are DBNPA and isothiazoline. These biocides help alleviate the microbiological activity on the membrane surfaces and the feedwater spacer, where bacteria are easily attached, thus reducing the potential of bacteria entering the system and feeding on the organics.
Chlorine is typically measured with an ORP in-line meter or a chlorine analyzer prior to entering the RO. It is a good practice to wet test for chlorine in addition to using on-line instrumentation.
Turbidity
Turbidity below 0.5 Ntu is a good target for makeup water, but low turbidity does not necessarily indicate the reduction of fouling potential; SDI testing provides more reliable insight.
If it is suspected that colloidal particles are entering the RO, run an SDI pad until it is plugged up and send the pad to the laboratory for a scanning electron microscope analysis. This test will indicate the characteristics of the atomic elements on the SDI pad, providing insight into what is fouling the RO.
Chemical Oxygen Demand (COD)
COD is found in gray water or industrial reuse and can be a major nutrient source for microbio.
A COD range of 8–10 mg/L is a good indication that the makeup source will have low fouling potential from COD.
Microbiological Matter
Two types of bacteria can be present in ROs: aerobic and anaerobic.
Aerobic bacteria
Classified by two types: planktonic and sessile
- Planktonic: free swimming
- Sessile: mature biological slime
Sessile bacteria are developed when aerobic bacteria are allowed to multiply and begin adhering to piping and membrane surfaces. This results in the aforementioned “flypaper” effect.
Best practice is to maintain microbio at 100 Cfu/mL in the feedwater and 1,000 Cfu/mL in the RO reject.
Anaerobic bacteria
- Typically found in ROs using gray or industrial reuse makeup.
- Iron-, denitrifying-, and sulfate-reducing bacteria are strong indications that mature biofilm has fully developed and microbiological control has been compromised.
Gases
Hydrogen Sulfide (H2S)
Normally, H2S is found in wells and is notable for its strong rotten egg odor. If oxidized or exposed to air, H2S can form elemental sulfur in the lead membrane, leading to potential blockage. To maintain system efficiency, the maximum level of H2S in RO feedwater is <0.1 ppm.
Hydrogen sulfide must be tested for on-site.
To test for H2S, fill up a water bottle halfway with makeup water, add a couple drops of hydrochloric acid into the bottle, shake, take the cap off, and smell. If hydrogen sulfide is present in the water, the odor will be apparent.
Carbon Dioxide (CO2)
As previously mentioned, the lower the alkalinity, the higher the CO2 content. Fortunately, CO2 does not play an active role in RO scaling or fouling. It passes directly through into the permeate.
It is important to monitor pH and alkalinity because they provide an indication of how much CO2 is flowing through the RO membrane. High amounts of CO2 can place a large load on the strong base anion and mixed bed.
Ammonia (NH3)
Ammonia is typically found in gray or industrial reuse makeup streams.
At a pH below 8.5, 90% or more of the ammonia is in its ionic form as NH4– and will be rejected in the RO like a monovalent ion. Above pH 9.5, ammonia becomes gaseous.
When mixed with TOC or phosphates, ammonia becomes suitable food for bacteria.
To test for ammonia, grab a water sample, add a few drops of 50% caustic, and shake the sample. If ammonia is present in the water, the strong smell will be apparent.
A Summary of RO Makeup Water Sources and Analysis Techniques
Surface Water
When using surface water for makeup, testing for microbio, organics, suspended solids, and colloidals is recommended. Season changes and shifts in weather conditions can cause rapid fluctuations in the levels of these components, so it is important to test frequently.
Well Water
Though well water quality is generally considered consistent, semi-annual testing is recommended, as it can have high mineral and hydrogen sulfide levels as well as some bacteria.
Gray Water
Various foulants in gray water may cause issues in RO systems, so frequent testing is recommended. It is important to test for bacteria and wash the cartridge filter housing regularly.
The Importance of Testing RO Makeup Water
Regardless of the makeup water source, we recommend trending water chemistry and other constituents and keeping trends on file. This data can assist in troubleshooting if RO problems arise.
As with all technology, due diligence is important to determine the feasibility of utilizing the methods outlined here. Always consult your equipment manuals and seek guidance from your water treatment representative to address plant-specific needs.
The post RO Makeup Water Sources: Addressing Variable Water Quality to Maintain System Efficiency appeared first on ChemTreat, Inc..
]]>The post Fractionation Plant Reduces Cooling System Biofouling with SurfClean™ 2.0 appeared first on ChemTreat, Inc..
]]>Process leaks in a fractionation plant’s cooling water system were leading to increased microbiological growth, necessitating increased amounts of bleach feed to maintain control. The fill in one tower was so plugged up with biological fouling that water and air flow were significantly impacted.
The quality of the makeup water used in the cooling towers presented an additional treatment challenge. The water contained hydrocarbons and microbes, and the ORP could reach as low as -200 mV, making it more difficult to treat effectively.
The Solution
Seeking to improve bleach performance and reduce the microbiological fouling occurring in the system, the facility began a trial of ChemTreat’s patented SurfClean 2.0, an innovative solution designed to address microbial fouling issues.
The local ChemTreat team set up a pump with a timer to slug feed SurfClean 2.0 twice per day. The team used a Teflon tee to mix bleach and SurfClean 2.0 at the optimum ratio and pumped the mixture into the tower basin through a short 10-foot tubing run.
tee to mix bleach and SurfClean 2.0 at the optimum ratio and pumped the mixture into the tower basin through a short 10-foot tubing run.
The facility was able to eliminate the feed of separate antifoam and surfactant treatments, as both are contained within the SurfClean 2.0 formula.
The Results
At the start of the SurfClean 2.0 treatment program, the tower screens required daily cleaning to remove biofouling released from the tower surfaces, which had accumulated during the previous treatment program.
At the beginning of the trial, SurfClean 2.0 flushed out biofouling accumulated during the previous treatment program.
After three months of using SurfClean 2.0, the air flow in the cooling towers increased, the water flow through the tower fill was more uniform, and biological growth in the fill was significantly reduced.
While the cooling water routinely contains 0 ppm of free chlorine because of process leaks, the total residuals are robust, with over 1.3 ppm of total chlorine being a typical reading. Dip slides used to monitor microbiological fouling showed no growth (<102 cfu/mL).
The poor makeup water quality in this system presented significant treatment challenges, but the application of SurfClean 2.0 resulted in improved water flow through the tower and reduced microbiological populations in the system while cleaning the tower fill.

Back corner of the cooling tower basin before the trial.

Back corner of the cooling tower basin two months into the trial. The water fall and wind circulation improved after SurfClean 2.0 application, indicating cleaning of the cooling tower fill.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Fractionation Plant Reduces Cooling System Biofouling with SurfClean™ 2.0 appeared first on ChemTreat, Inc..
]]>The post Industrial Complex Saves Over $7M with ChemTreat Treatment Program and Intelligent Water Management appeared first on ChemTreat, Inc..
]]>In 2013, ChemTreat initiated a water quality evaluation for a 22,000-gpm river water clarification system supplying water to an industrial complex in the Southern U.S.
The following challenges were identified during the evaluation:
- The existing inorganic coagulant/caustic program did not provide optimal total suspended solids/total dissolved solids (TSS/TDS) control for the influent river water.
- Historical effluent quality issues caused by ineffective clarification resulted in high TSS, aluminum post precipitation, and high colloidal silica in the treated water. This then created performance and reliability issues throughout the facility.
These issues caused plant management to consider capital replacement of the water plant at an estimated cost of $30M.
The Solution
After a thorough system survey, ChemTreat developed and implemented an action plan to address water quality issues in a cost-effective manner with a focus on continuous improvement, innovative automation, training, and data analysis.
Highlights of the improvement plan and treatment program included:
- Coagulant chemistry upgraded and caustic usage eliminated, lowering chemical costs and improving color and turbidity removal, and overall quality to record levels.
- Sludge generation reduced by 90%.
- Regular operator trainings implemented to empower operators to identify and mitigate water quality issues.
- Special projects completed, including a demineralizer optimization study, chemical feed automation, water balance, distribution system corrosion control, and power condenser efficiency modeling.
- ChemTreat’s CTVista®+ intelligent water management platform integrated into the plant’s water system to collect and analyze water plant data, allowing for data dissemination to plant management via daily scorecards.
- Standard operating procedures, control ranges, and testing procedures updated with plant management.
- Weekly project meetings with plant management adopted to identify and implement key projects for improving operations and reducing total costs.
The Results
The extensive water management program was developed, implemented, and continues to be overseen by the local ChemTreat team and Polymer Technical Staff, with a combined 220 years of water treatment experience.
ChemTreat’s customized and innovative approach to the facility’s water treatment program achieved substantial savings for the customer:
$30M in capital savings (estimated cost of replacing the water plant)
$1.3M in annual savings
$7.2M total savings from 2017 to 2023
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Industrial Complex Saves Over $7M with ChemTreat Treatment Program and Intelligent Water Management appeared first on ChemTreat, Inc..
]]>The post Chemical Processor Reduces Water and Chemical Usage with Custom Chlorine Dioxide RO Cleaning Program appeared first on ChemTreat, Inc..
]]>A chemical processing facility on the Gulf Coast used bleach, bisulfite, and DNBPA to slow biofilm growth on the membranes in their reverse osmosis plant.
The high organic content and the alkaline pH of the supply water resulted in high disinfection chemical feed rates.
Typical run times between membrane cleanings was 2–4 weeks, using a lot of RO permeate and cleaning products and contributing to a high level of trihalomethanes (THMs) leaving the RO system.
The Solution
The facility worked with their local ChemTreat team to develop a chlorine dioxide (ClO2) program to replace bleach and DNBPA treatment at the RO plant.
The new treatment program significantly reduced the amount of bleach, DNBPA, bisulfite, and RO cleaning chemicals needed to maintain membrane cleanliness and extend run times. Cleanings now only take place once every 3 months.
The Results
Thanks to the new ClO2 program and the subsequent reduction in membrane cleanings, the facility was able to cut treatment chemical usage by almost 80%.
Permeate used for membrane cleanings was reduced by 50%.
Implementing the ClO2 program has resulted in the following annual savings associated with membrane cleaning:
- 144,000 gallons of permeate
- 85,000 gallons of treatment chemicals
- $550,000 in approximate annual costs
Manpower requirements and waste generation were reduced thanks to the decreased frequency of cleanings.
The enhancement of the treatment program also resulted in an 80% reduction of THMs (disinfection byproducts) leaving the RO system, helping the plant achieve their environmental goals.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Chemical Processor Reduces Water and Chemical Usage with Custom Chlorine Dioxide RO Cleaning Program appeared first on ChemTreat, Inc..
]]>The post Manufacturing Facility Saves on Chemical Costs and Water Usage with Demineralizer Improvements appeared first on ChemTreat, Inc..
]]>A manufacturing facility in the Southwestern US runs city water through a series of zeolite softeners, multi-media filters, activated carbon, reverse osmosis, and four mixed bed demineralizers.
The demineralizers were not operating at their full capacity, leading to poor regeneration and impacting the facility’s efficiency and production.
After observing a regeneration in one of the demineralizers, the ChemTreat team identified several issues, including:
- Incorrect resin levels
- High backwash flow rates
- Elevated levels of caustic and acid usage
- Lack of visible separation of the anion and cation resins after backwash and settling
The Solution
After a thorough inspection, the local ChemTreat team provided a list of recommendations to improve demineralizer operations.
- Program the control system to adjust flow for each step of the demineralization process.
- Replace resin or separate it to determine the correct amount of anionic and cationic resin in all four vessels.
- Adjust acid and caustic pumps to feed the correct amount of each during regenerations.
- Repair the air flow rotameter.
- Develop a log sheet to allow operators to routinely audit regenerations, monitoring air and water flow, valve positions, anion/cation separation, acid and caustic concentrations, and rinse down conductivity.
The Results
Based on ChemTreat’s recommendations, facility management took the following steps to improve demineralizer operation:
- Purchased new resin.
- Performed extensive repairs on the vessels to reduce resin loss.
- Added resin to the beds and regenerated the resin manually.
- Correctly programmed the water flow automation process.
These improvements dramatically improved the function of the mixed bed demineralizers.
Estimated Annual Savings from System Repairs and Enhancements
$71k in caustic and acid costs
651K gallons of water required for regenerations
282 hours of operator time needed for regenerations and rinses
64% reduction in chemicals pumped into the facility
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
Featured Resource

ChemTreat’s Membrane Laboratory
ChemTreat’s state-of-the-art reverse osmosis laboratory located in San Diego, California offers a complete array of laboratory services to improve membrane performance and develop new formulations for specific applications. The lab performs membrane autopsies, which include in-depth analytical testing, results interpretation, reporting, and recommendations.
Read MoreThe post Manufacturing Facility Saves on Chemical Costs and Water Usage with Demineralizer Improvements appeared first on ChemTreat, Inc..
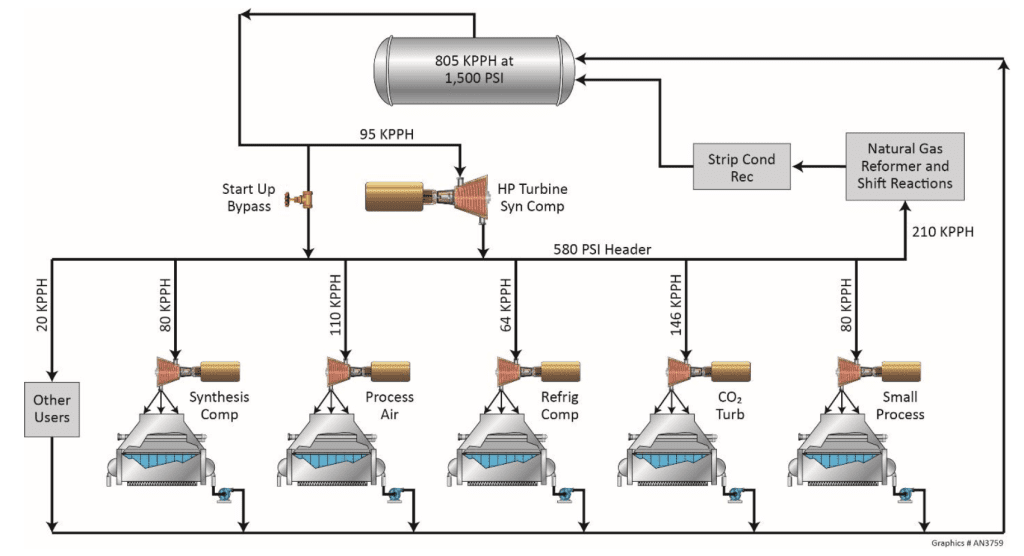
]]>The post Minimizing Corrosion and Deposition in High-Pressure, High-Purity Steam Generators for Chemical Processors appeared first on ChemTreat, Inc..
]]>This article provides:
- 1. A basic overview of high-pressure, high-purity steam cycle chemistry for the chemical, petrochemical, and refining industries
- 2. An introduction to the four pillars of steam cycle treatment
- 3. A discussion of typical steam generator issues at chemical processing plants, as well as opportunities for improvement
- a. Anionic and cationic impurities
- b. Corrosion and condensation
- c. Lack of steam quality monitoring and instrumentation
We hope this discussion will help chemical facility personnel improve the long-term production and reliability of their steam systems.
Why Boiler Water Treatment Matters
An Overview of High-Pressure, High-Purity Steam Cycle Chemistry
The primary purpose of boiler water treatment is reducing corrosion throughout the steam cycle.
Treating boilers differs slightly from cooling tower, closed loop, and other chemical system treatment. For instance, true corrosion inhibitors are not added to a boiler system. Instead, aggressive anions like calcium, magnesium, chlorides, sulfates, and silicas are removed.
Oxygen is also removed via mechanical and chemical means.
Higher pH and temperatures stimulate the natural passivation processes that reduce corrosion and emissions, so boiler treatment typically involves raising the pH as well.
The secondary purpose of boiler water treatment is reducing deposition following pretreatment.
Iron and, to a lesser degree, copper deposition is particularly problematic, as it can transport into steam generators and boilers. Iron transported into boilers deposits in high heat flux areas, potentially leading to underdeposit corrosion.
The Four Pillars of Steam Cycle Treatment
The four pillars of steam cycle treatment are a useful tool for understanding boiler treatment and the common gaps seen at chemical processing facilities.
In descending level of importance, the pillars are:
- 1. High-quality feedwater production
- 2. Condensate and corrosion reduction
- 3. Internal treatment selection and AVT
- 4. Dispersant treatment for metal transport
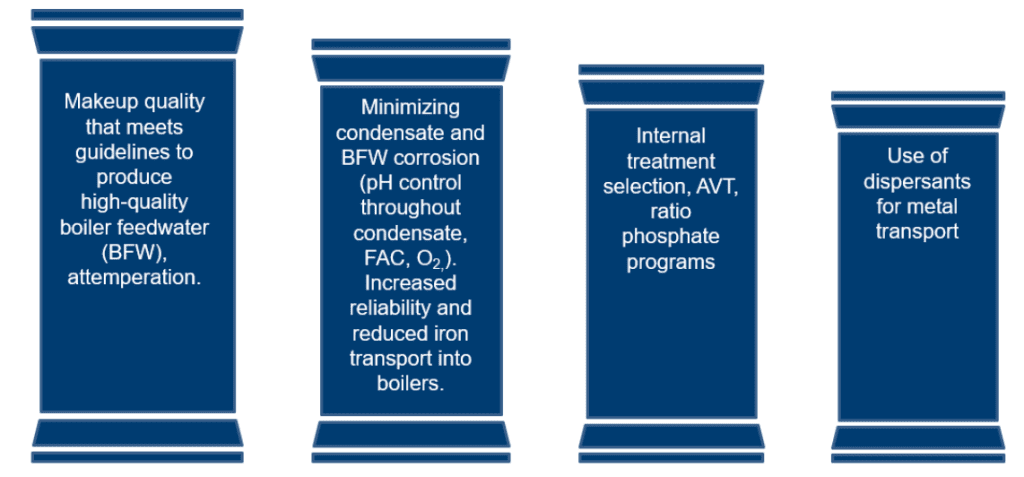
It is important to note that:
- If the first two pillars are addressed with minimal gaps, the second two pillars become less problematic.
- If there are gaps or failures in the first two pillars, the second two similarly matter less, as system reliability issues become more prevalent.
Pillar 1: Producing High-Quality Feedwater through High-Quality Makeup
The first pillar represents a particularly challenging issue in the chemical processing industry. High-quality feedwater is created through the attemperation of high-quality makeup, otherwise known as boiler feedwater (BFW). This process involves controlling and measuring low-level ionic impurities to avoid boiler feedwater contamination, as contaminants can find their way into the steam through the attemperation process.
Pillar 2: Minimizing Condensate and Boiler Feedwater Corrosion
The second pillar focuses on minimizing condensate and BFW corrosion. This is typically accomplished by applying amines or ammonia, as well as through oxygen scavenging.
Generally, the goal is to minimize corrosion products and iron transport through the system. More specifically, we will explore flow-accelerated corrosion (FAC), another major gap faced by chemical and petrochemical plants as well as refineries.
Pillar 3: Selecting the Appropriate Internal Treatment
The third pillar addresses the importance of selecting the right steam gyrator and boiler internal treatment based on a system’s specific needs. This could include all-volatile treatment (AVT) ratio programs using sodium phosphate or all-polymer programs that can be used at lower pressures.
Pillar 4: Using Dispersants for Metal Transport
The last pillar refers to using polymers to disperse iron. In certain instances, this treatment is recommended for reducing iron transport into steam generators and boilers.
Three Common Steam Cycle Treatment Gaps in Chemical Processing Facilities
The four pillars provide a solid foundation for a deeper discussion into the specific challenges many chemical, petrochemical, and refining facilities face.
- 1. Anionic and cationic impurities
- 2. Accelerated corrosion and condensate
- 3. Lack of steam quality monitoring and instrumentation
1. Anionic Impurities and Cationic Impurities
Feedwater quality is a major area of improvement for many chemical processors. In fact, demineralized water quality drives the majority of boiler chemistry needs.
Unfortunately, many plants do not follow best practices and operate boilers with low levels of ionic impurities in their makeup water. Controlling and measuring low-level ionic impurities is an important factor for maintaining boiler efficiency.
Cation Slipping
Plants typically use some form of mixed bed ion exchange to produce higher purity water.
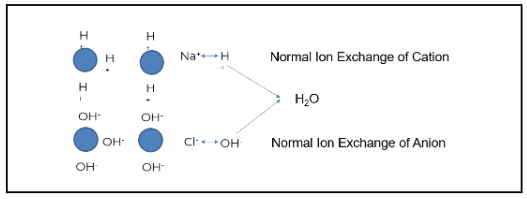
The image above demonstrates how this process works when ion exchange is properly generated and operating as intended. The blue circles represent ion beads. When regenerated, the cation is loaded with a hydrogen or hydronium ion. The anion bead is loaded with a hydroxyl ion.
Using the example of salt (NaCl) and water (H2O), sodium (the cation) exchanges with a hydrogen ion. Chloride (the anionic portion) exchanges with a hydroxyl ion, creating pure water. Under the right conditions, regeneration produces high-quality feedwater. However, gaps such as cation bed slipping can occur when the ion exchange does not operate as designed.
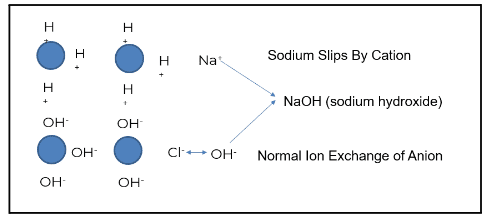
Sodium is a prevalent ion seen when the cation bed starts to slip. In the example above, sodium does not exchange with the hydronium or hydrogen ion; this is known as “slipping.” The slip causes the sodium to go through the chloride. Sodium chloride then exchanges out with a hydroxyl ion, and sodium slips as sodium hydroxide.
The sodium hydroxide will first concentrate in the boiler water, driving up boiler pH. This will increase the sodium-to-phosphate ratio, which, in turn, increases the risk of underdeposit corrosion and caustic gouging. Attemperation contamination reduces the steam quality for turbines, and calcium and magnesium can pass under more stressed conditions.
Even slightly elevated sodium levels pose a risk of sodium hydroxide formation in iron deposits. When this occurs, porous iron deposits in boilers (known as “wick boiling,” which will be discussed later in this article), the boiler water evaporates off, and high levels of iron concentrate remain.
If sodium hydroxide reaches the feedwater, which is normally used for de-superheating, it could directly contaminate the steam going to the turbine blades, reducing efficiency. Hydroxide can also deposit onto the turbine blades in the phase transition zone as it starts to condense, potentially causing stress corrosion cracking, usually on the roots, where the turbine blades attach to the rotor.
Under extreme conditions, as the cation resin is exhausted, the hardness ions of calcium and magnesium will start to pass, which can cause deposit issues in boilers at higher temperatures. Boilers are virtually intolerant to hardness at this level, and calcium magnesium can react with the alkalinity and start to drop out pH in the boiler.
Anion Slipping
Though not as common as cation slipping, anion slipping is an important point to discuss. Interestingly, slipping anions has the opposite effect of a slipping cation. The first anion we typically see slip is silica. Slipping silica combines with the exchanged hydronium ion, forming a slightly acidic species. Depending on the temperature, pressure, and pH, silica in these high-pressure, high-purity boilers is prone to mechanical and vaporous carryover in drums. This poses a risk to turbines; silica forms deposits on turbine blades, reducing efficiency.
Another problem with silica is that, unlike sodium, it cannot be removed with water washing. Silica forms a very tenacious deposit on turbine blades that is difficult to remove.
Other anions that can slip are chlorides and sulfates, which combine with the hydrogen ion to form mineral acids, such as hydrochloric and sulfuric acid, driving down boiler pH. These increase the risk of underdeposit corrosion in porous iron deposits.
If mineral acids enter the steam, they provide another mechanism for stress corrosion cracking in turbine blades.
2. Accelerated Condensate and Corrosion
When it comes to minimizing condensate and corrosion, the main priority is reducing levels of iron and other metal transport into the boilers.
Iron and Copper Transport into Steam Generators
Water systems in chemical processing facilities tend to be large, with complicated condensate systems and extensive steam cycles. Typically, the feedwater going into the boiler will have elevated iron levels, or even yellow metals. Any metal entering the boiler may deposit on boiler surfaces; it is difficult to keep them in solution in higher heat flux areas. This creates potential sites for underdeposit corrosion.
The following images show examples of underdeposit corrosion. The first is a transfer line exchanger (TLE) from a methanol plant, where waste heat gas was sent through the tubes. Acid phosphate wastage caused the formation of underdeposit corrosion sites. The plant had switched to a much higher-level sodium phosphate program years before, but the damage was already done.
The following images show examples of underdeposit corrosion. The first is a transfer line exchanger (TLE) from a methanol plant, where waste heat gas was sent through the tubes. Acid phosphate wastage caused the formation of underdeposit corrosion sites. The plant had switched to a much higher-level sodium phosphate program years before, but the damage was already done.

This represents a major challenge of underdeposit corrosion: once it occurs, bulk water chemistry will have minimal effect on existing underdeposit corrosion sites.
The second image is an example of a thick wall failure in a utility boiler. It shows an entire blowout, known as hydrogen damage. This was caused by a very acidic species depositing within the steel matrix and converting the carbon sites over to methane, leading the wall to expand and eventually resulting in the large failure seen here.
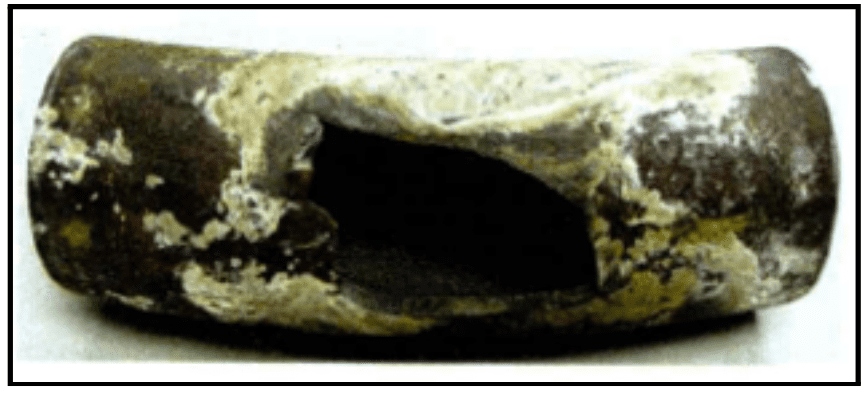
Image courtesy of Kurt Kraetsch, ChemTreat
These images illustrate the importance of pretreatment to boiler efficiency. Effective pretreatment reduces iron and other metal transport into the preboiler. Approximately 30% of iron entering a boiler remains soluble and is taken out via continuous blowdown.
Polymer treatment can help reduce metal transport and may be a good option for boilers with a history of underdeposit corrosion issues.
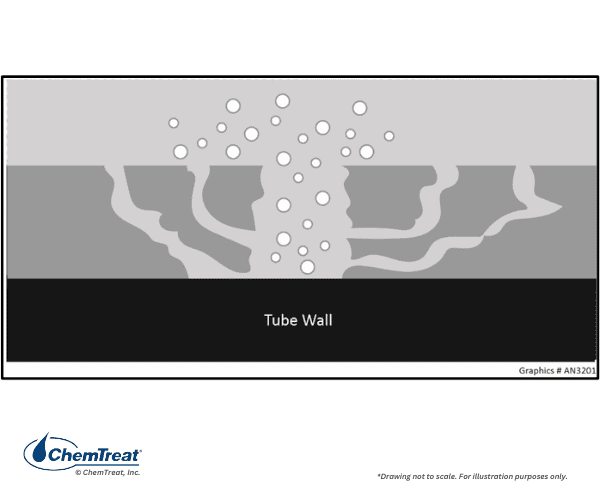
Wick Boiling
The following graphic demonstrates a principle called wick boiling, where porous iron deposits form on a boiler tube’s higher heat flux areas. These deposits occur when water with ionic impurities flows into the tube and evaporates off, creating high levels of concentration of these acidic and caustic species. This results in underdeposit corrosion in the form of tube wastage. Wick boiling typically causes hydrogen damage at a pH of 4, acid phosphate at low pH, and caustic gouging at high pH.
Balancing Mild Steel and Copper Corrosion in the Preboiler
Mixed-metallurgy boilers pose additional water treatment challenges. These boilers are typically made with mild steel and some form of yellow metal, such as copper and copper alloys like nickel and brass.
Copper and steel require completely different types of treatment to inhibit corrosion, which makes it difficult to calibrate treatment accordingly.
For copper, corrosion inhibition usually involves lowering pH to the 7–8 range. Higher levels of oxygen scavenger are typically added to accomplish this.
Mild steel, on the other hand, responds better to amines and a higher pH.
Balancing pH and amine/ammonia and oxygen scavenger feed is a constant challenge in mixed metallurgy systems.
Flow-Accelerated Corrosion: Feedwater and Condensate
Controlling flow-accelerated corrosion (FAC) presents one of the most significant gaps in water treatment for the chemical industry. FAC occurs when the rate of oxygen, or the rate at which the magnetite layer dissolves, is greater than its rate of formation.
Though this concept has long been well-understood, best practices for its mitigation has not been consistently applied in the chemical industry.
The following graphic illustrates the FAC cycle.
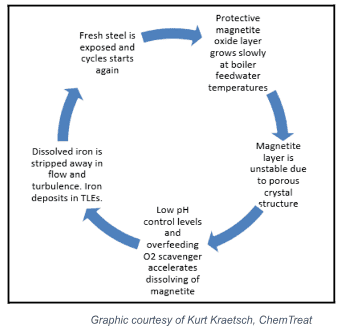
The process begins in the upper right-hand corner. The oxide layer grows slowly at first, as boiler feedwater temperatures are mild.
The magnetite layer continues to grow, developing an unstable, porous crystal structure.
Controlling ammonia/amine and oxygen scavenger comes into play at the next stage, as lower pH and high oxygen scavenger levels accelerate the dissolution of the magnetite layer.
As the iron is dissolved, it is stripped away in high-flow and turbulent areas, forming deposits in TLEs and boilers.
This leaves the fresh metal exposed, restarting the cycle.
Reducing FAC Potential with Feedwater pH Control
The graphic below shows the solubility of the magnetite layer, representing the risk of FAC.
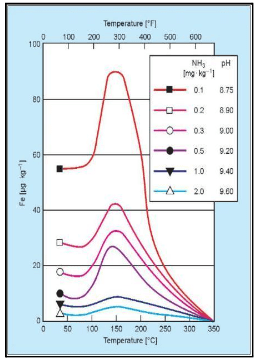
The horizontal axis displays the temperature, while the vertical axis correlates to solubility. The risk peaks at approximately 300°F, or 150°C, which roughly corresponds to standard feed system operation, depending on deaerator pressure.
The individual-colored lines represent pH. As pH increases, the system’s ability to resist the dissolution of the magnetite layer increases. The highest solubility shown on this graph is a pH 8.75, dropping off significantly as pH increases to the 9.4–9.6 range. Thus, balancing feedwater and condensate operation at the highest practical pH, while effectively treating yellow metals, can be very difficult.
Understanding the Impact of Oxygen on FAC
The impact of oxygen on FAC is not well understood. Oxygen works synergistically with aggressive anions like chlorides and sulfates, causing corrosion. However, oxygen can also help reduce FAC.
As previously mentioned, the magnetite layer forming in feedwater systems is porous. If the gaps in the layer are filled with hematite by slightly increasing oxygen ingress, a stronger oxide layer will develop, which is less likely to dissolve and cause FAC.
In all-steel systems, pH is not always set as high as necessary, and reducing or removing oxygen scavenger application may help reduce FAC, depending on iron transport analysis.
3. Lack of Steam Quality Monitoring and Instrumentation
As with the improvement opportunities discussed in the previous section, following best practices around monitoring and instrumentation in steam systems is an important factor of effective water treatment.
Monitoring steam quality helps maintain the purity of the steam going to the turbines. Some common monitoring parameters are reviewed in the next sections, followed by a discussion of the benefits of using instrumentation over relying solely on sampling.
Sodium and Silica
Sodium and silica deposits, as well as aggressive anions like chlorides and sulfates, can cause stress corrosion cracking at the wet ends of turbine blades, reducing system efficiency.


These analytical reports show the presence of various impurities in a 600-pound ethylene unit during standard operations versus a condenser leak. It is important to note the conductivity showed very little change during the condenser leak. This facility used ultra-low-level wet-based testing to track impurities but did not have sodium monitoring in place. Using instrumentation to monitor sodium may have caught the condenser leak earlier.
Monitoring feedwater and steam with instrumentation is a water treatment best practice. However, these systems may be difficult and expensive to retrofit and maintain.
One alternative is monitoring cation conductivity, which provides a good option for testing low-level ionic impurities.
Cation Conductivity Monitoring
There are two primary types of cation conductivity monitoring.
- 1. Degassed cation conductivity, where the water is boiled off, then cooled back down and run through a strong cation exchange column, typically to remove CO2. Newer units may use a nitrogen purge to remove CO2.
- 2. Un-degassed cation conductivity monitoring, in which water is run through the cation exchange column without being boiled off.
Photo courtesy of Hach.
Regardless of whether water is degassed, the key is to measure the conductivity before and after the cation exchange column.
Two Principles of Cation Conductivity Monitoring
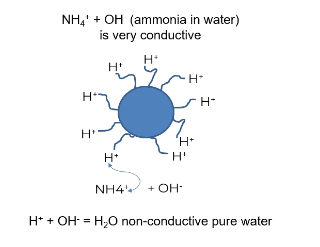
- 1. The majority of conductivity found in steam, feedwater, and condensate systems comes from adding ammonia or amines, not from ionic impurities. Monitoring cation conductivity removes the impact of ammonia or amines on the conductivity reading. The diagram below shows ammonia as ammonia hydroxide in water. The ammonium portion (the cation) will exchange out with a hydronium or hydroxyzine to form pure water, taking out the masking effect produced by amines or ammonia.
- 2. Cation conductivity converts low levels of impurities to acids, which are always more conductive than their neutral salts. During a condenser leak or a period of poor ion exchange, sodium chloride is a typical impurity. The sodium in sodium chloride exchanges out with a hydronium hydroxyl or hydrogen ion to form hydrochloric acid, amplifying the conductivity’s effect by taking low part per billion levels of ionic impurities and converting them into acids.
- a. During the ethylene unit condenser leak shown previously, there was a slight uptake in normal, un-neutralized, or non-cation, conductivity. The average conductivity increased from 3–4.5 to 3.5–4.5. It would typically be difficult to note this difference; however, the cation conductivity shot up by a factor of 10In areas such as feedwater, steam, and condensate systems, cation conductivity offers an efficient way to measure and monitor very low levels of ionic impurities via instrumentation.
The Benefits of Instrumentation Over Sampling
The example above illustrates the importance of instrumentation in helping facilities catch boiler system issues.
Some chemical processing plants still rely solely on “grab” samples, which provide an incomplete picture of the system water quality. Particularly in high-pressure, high-purity units, monitoring with instrumentation is best practice, with grab sampling used only as a backup.
Conclusion
Minimizing corrosion and deposition in high-purity, high-pressure steam generators and boilers is an important component of maintaining system reliability and efficiency in chemical, petrochemical, and refining facilities.
When designing a water treatment program, it is important to keep the four pillars of steam water treatment in mind, as well as identifying areas of improvement based on common gaps.
The ultimate goal of this post was to address these gaps in high-purity, high-pressure steam generator treatment and help your facility improve the long-term reliability of your boilers and steam turbines by implementing best practices around treatment chemistries and monitoring.
As with all other technologies, due diligence is necessary to determine the feasibility of utilizing the methods discussed in this post. It is important to consult your equipment manuals and guides and seek guidance from your local water treatment representative to address the specific needs of your facility.
The post Minimizing Corrosion and Deposition in High-Pressure, High-Purity Steam Generators for Chemical Processors appeared first on ChemTreat, Inc..
]]>The post Refinery Saves $90K and 5.6M Gallons of Water Annually with RO Monitoring Enhancements appeared first on ChemTreat, Inc..
]]>A refinery on the Gulf Coast was experiencing performance issues in their reverse osmosis (RO) system. The RO was struggling to provide sufficient water to run through the boilers, and any further decreases in performance would have required the refinery to reduce production rates.
The Solution
The local ChemTreat team began inspecting the refinery’s RO units monthly to track issues and identify improvement opportunities.
During one of these inspections, the ChemTreat team noted a dramatic increase in the permeate conductivity in several pressure vessels. Refinery personnel repaired the vessels, lowering the total conductivity from 122 to 52 µmhos, a 57% decrease.
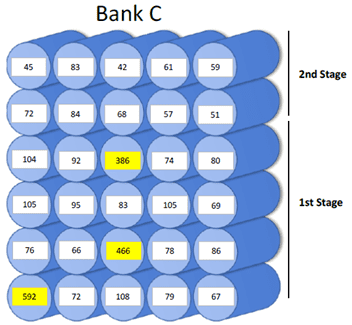
Diagram showing the pressure vessels with high conductivity.
During the same inspection, the RO permeate tank conductivity decreased by 51 µmhos in just three days, showing the positive impact of repairs on water production capability.
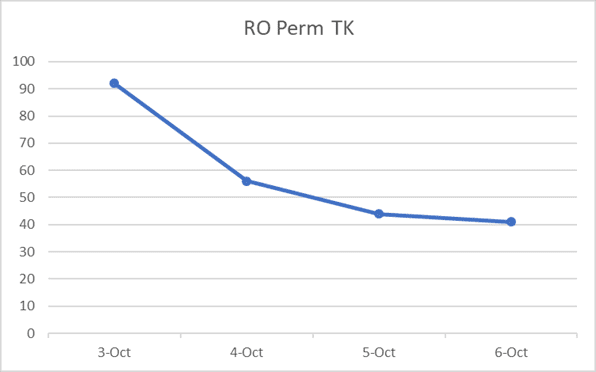
Trend chart illustrating the dramatic decrease in permeate tank conductivity.
The Results
By quickly taking action to repair the pressure vessels, refinery operators significantly improved demineralizer throughputs, saving on acid and caustic costs. The reduction in water used for regenerations additionally saved on treatment costs for water being sent to the sewer.
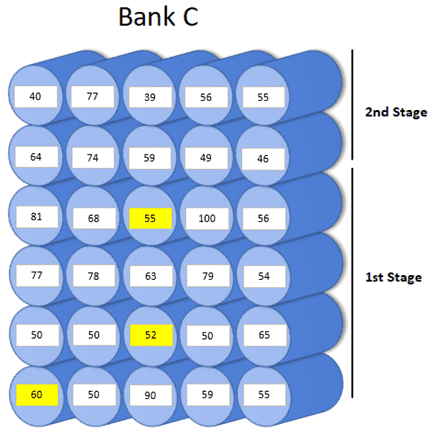
Diagram of pressure vessel conductivity after repairs were made.
Trend chart tracking demineralizer throughputs, which is heavily influenced by RO permeate quality. The yellow line indicates the date of repairs, with throughputs increasing after repairs were completed.
The RO system improvements made after ChemTreat’s inspection yielded the following benefits to the refinery:

$90,000 in approximate annual savings on regenerant chemical costs

57% decrease in total RO conductivity
5.6 million gallons less regeneration water being sent to the sewer annually
Results are examples only. They are not guaranteed. Actual results may vary.
The post Refinery Saves $90K and 5.6M Gallons of Water Annually with RO Monitoring Enhancements appeared first on ChemTreat, Inc..
]]>The post Major Hospital System Improves Cooling System Monitoring and Control with Smart Release® Second Generation Solid Treatment Technology appeared first on ChemTreat, Inc..
]]>A major hospital system on the East Coast runs a 2,400-ton chiller as part of its cooling system. The chiller water was treated with conventional solid technology, which lacked the capability to monitor and control chemical feed and manage inventory.
As part of their Water Safety Management program, the customer was looking for a way to control the dosing of solid oxidizing biocide to maintain optimum biocontrol in their cooling system.
The Solution
ChemTreat collaborated with Dober to install the second generation of Smart Release (SRG2) solid cooling treatment technology at the customer site.
An SRG2 skid with three solid product feeders was installed, along with an automation panel for control and monitoring. The panel has the capability to track key performance indicators such as conductivity, PTSA, pH, and ORP.
SRG2 skid installed at the customer site, including enhanced automation and control for product dosing capability
Additionally, a fourth feeder was included in the skid for supplementing azole-based corrosion inhibitor. An ORP probe-based biocide dosing capability was provided to meet the customer’s treatment needs.
A fourth feeder was installed in the skid to supplement the azole-based corrosion inhibitor
The Results
KPI trends were collected and shared through daily CTVista®+ reports, and bi-weekly water sampling analyses reports were tracked for performance tracking purposes.
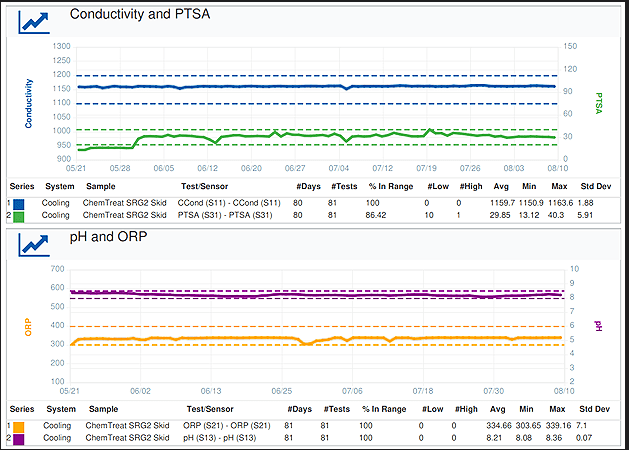
Cooling system trends from the customer’s daily summary report generated in CTVista+
The installation of the SRG2 skid helped control the product dosing through PTSA. The ORP-based biocide dosing showed good microbiological control in the cooling water.
This new solid cooling treatment technology allowed the customer to control product dosage more precisely, successfully reducing microbiological fouling.
Download the Case StudyResults are examples only. They are not guaranteed. Actual results may vary.
The post Major Hospital System Improves Cooling System Monitoring and Control with Smart Release® Second Generation Solid Treatment Technology appeared first on ChemTreat, Inc..
]]>The post Paper Mill Reduces Caustic Usage and CO<sub>2</sub> Emissions with ChemTreat Reverse Osmosis Solutions appeared first on ChemTreat, Inc..
]]>The Problem
A paper mill in the Southeastern US uses water from several wells with high silica and total alkalinity. The well water is treated with a demineralizer system consisting of a cation unit, degasifier, and an anion unit. The anion unit required 15,000 pounds of caustic per day for regeneration. As the cost of caustic increased, the plant was looking for ways to economize.
The Solution
In addition to the demineralizer, the mill has a two-pass reverse osmosis (RO) unit capable of producing 500 gpm of permeate. The unit had been shut down because of fouling issues.
After surveying the RO and analyzing the well water, ChemTreat proposed a cost savings project to reduce caustic usage by 26%.
The Results
Reactivating the RO system and changing it from a two-pass setup to two separate units allowed the plant to produce 950 gpm of permeate to blend with the well water. Having two ROs in operation also extended the demineralizer’s throughput from 1.2 to 1.62 million gallons, reducing the number of daily regenerations from 4.32 to 3.2. This resulted in a daily savings of 3,920 pounds of caustic, or 1.43 million pounds per year.
Reactivating the RO units also reduced the total alkalinity in the degasifier, eliminating 1,311 pounds of carbon dioxide per day, contributing to the plant’s environmental stewardship goals.
- 450 gpm increase of permeate produced per day
- 420,000 gallons of demineralizer throughput added per day
- 1.43 million pounds of caustic saved per year
- 1,311 pounds of CO2 emissions eliminated per day
Results are examples only. They are not guaranteed. Actual results may vary.
The post Paper Mill Reduces Caustic Usage and CO<sub>2</sub> Emissions with ChemTreat Reverse Osmosis Solutions appeared first on ChemTreat, Inc..
]]>
